🎯The working substance is essential part of thermodynamic system by means of which we can study heat and mass transfer across system.
🎯As long as fluid remains homogeneous in composition and chemical form, and retains its chemical identity, it is known as pure substance.
🎯System is defined as ‘prescribed region of space or finite quantity of matter surrounded by an envelope called boundary’.
🎯”Space and matter external to the thermodynamic system and outside boundary is called as surrounding’.
🎯”When system and surrounding are put together it is called universe’.
🎯Different types of systems are: closed system, open system and isolated system.
🎯A system is called as closed system if mass within the boundary of the system remain constant and only energy may trarsfer across boundary.
🎯A system is called as open system if mass as well as energy transfer across the boundary.
🎯A system is said to be isolated system if no flow of energy and mass takes place across boundary of system.
🎯State of systern is defined as ‘exact condition of system’.
🎯Observable characteristic of system is known as property of system.
🎯Properties are of two type: Intensive property and Extensive property.
🎯Zeroth law: If two systems are each in thermal equilibrium with the third system, they are also in thermal equilibrium with each other.
🎯First law of thermodynamics: If system undergoes a cyclic change, then algebraic sum of work delivered to the surrounding is directly proportional to the algebraic sum of heat taken from the surrounding.
🎯Steady flow energy equation:
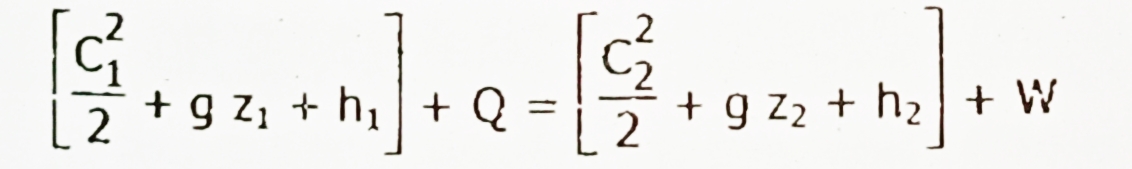
🎯Keivin and Clausius statements are eouivalent Viglation of orie will have violation of other.
🎯Perpetual motion machine of first and second kind is impossible.
- Diploma Mechanical Engineering Fourth sem books Pdf download …
- Practice tests for Mechanical Engineering Students (ME3I) . Revision for better understanding .
- Protected: “सतर्क भारत, समृद्ध भारत” week from 27 Oct – 2 Nov for Corruption free India…. Celebrated by Mechanical Engineering Department , Sanjivani K.B.P. Polytechnic Kopargaon .
- IMP Points for ME3I | Mechanical Engineering third sem
Leave a comment